What Is Osmosis Byju\'s
Is Sweating an example of osmosis. Osmosis can be defined as the movement of water from where it is in higher concentration to where it is in lower concentration through a semi-permeable membrane.
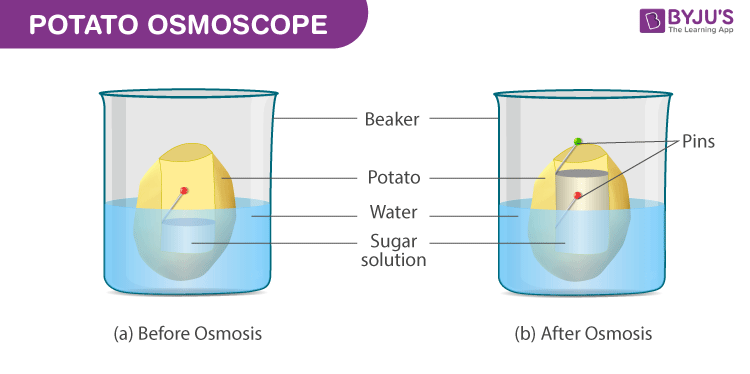
Study Of Osmosis By Potato Osmometer An Experiment
Get the answer to this question and other important questions asked in NEET only at BYJUS.

What is osmosis byju\'s. Osmosis can be defined as a flow of water from an area of low concentration of a solute to an area of higher concentration of a solute. Check this Answer for the question What does byjus provide. Now a Keep cup A empty.
In the diagram the concentration of sugar is initially higher on the right side of the membrane. They gave me some money and the mission to communicate science concepts back to the. Ions atoms and molecules dissolved in liquid constitutes to area of low concentration.
Know about byjus application how and when it started what are the benefits etc here. Put each potato cup in a trough containing water. The process of moving of solvent particles across a semipermeable membrane from a dilute solution into a concentrated solution to equalize concentration.
C Put one teaspoon salt in cup C. The factors affecting the rate of osmosis include. The flow of this solution stops when equalization happens on both sides of the membrane.
Osmosis is a biophysical process occurring commonly in biological systems where solvent molecules move across a semi-permeable membrane towards a region of high solute concentration. BYJUS - The Learning App is the common brand name for Think and Learn Private Ltd. In biological systems the solvent will usually be water.
Note that this reversed flow produces pure salt solution water because the membrane is. D Put one teaspoon sugar in the boiled potato cup D. This is called reverse abbreviated RO osmosis.
BYJUS - The Learning App is the common brand name for Think and Learn Private Ltd. The movement of water from high concentration to low concentration through a semi-permeable membrane is called osmosis. Here you can find answers for more than 5 Million questions.
BYJUS comprehensive e-learning programs for K3 K10 K12 NEET JEE UPSC Bank Exams from Indias best teachers. Osmosis will occur whenever the water concentrations are different on either side of a differentially permeable membrane. The complete process does not require energy in order to take place.
Osmosis is a natural process which describes the diffusion of water molecules through a semipermeable membrane from a lower concentrated solution to a higher concentrated solution. Your body doesnt pump water to your skin in. A simple and full explanation of osmosis and how does it work and a real application about this phenomenon.
To make it easier for you imagine there is a cup which has water in it. Keep these for two hours. Ask Sawal is a question answer discussion forum.
Raisins when kept in water swell up. Osmosis is the movement of a solvent such as water from a low solute concentration into a solution that has a higher solute concentration through a semipermeable membrane. B Put one teaspoon sugar in cup B.
I recently won a competition called Im a scientist get me outta here. One of these potato cups should be made from a boiled potato. What type of transport is osmosis.
A semi-permeable membrane is any membrane that allows only certain substances to pass through it. Osmosis is the diffusion of a solvent through a differentially permeable membrane. Your sweat glands use osmosis.
The simplest definition of a semipermeable membrane is one that lets the solvent water but not the solute eg. Osmotic pressure is a pressure that is required to be applied to solution in order to prevent an inner ward flow of a pure solvent across a semipermeable membrane. Osmosis and diffusion are the two different types of passive transport which play a vital role in moving molecules in and out of the cell.
On the contrary diffusion does not require a semi-permeable membrane to occur and the molecules move from a region of higher concentration to lower concentration. The process named Osmosis is the reason why the water from the surrounding medium enters the Cells. Osmosis is of two types- endosmosis and exosmosis.
Osmosis is the diffusion of water across a partially permeable membrane from a dilute solution high concentration of water to a concentrated solution low concentration of water. Osmosis is a process of movement of solvents through a semi-permeable membrane from a region of lower solute concentration to higher solute concentration. Osmosis is a process in which liquid water flows through a semipermeable membrane from a diluted solution into a more concentrated solution.
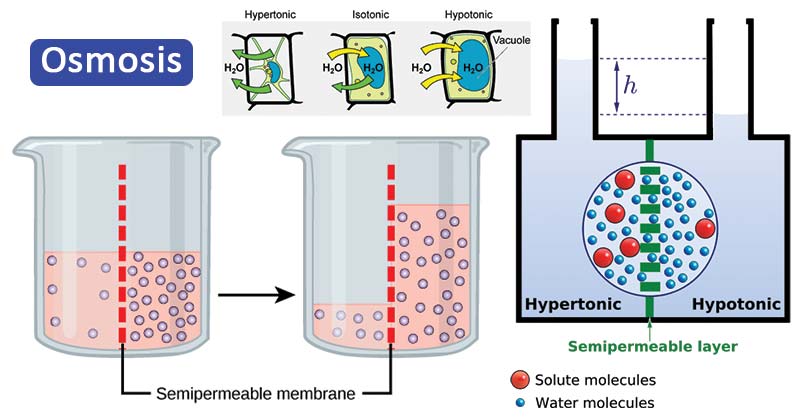
Osmosis Definition Types Examples Osmosis Vs Diffusion

Thermotropism Study Flashcards Plants Roots Show
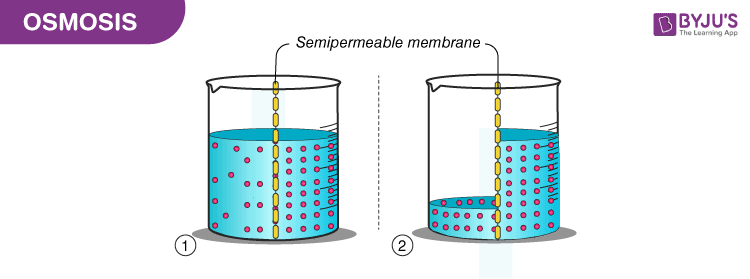
What Is Osmosis Definition Types Osmotic Pressure

Diffusion In Plants Diffuser Passive Transport Cells And Tissues

Green Chemistry 12 Principles Of Green Chemistry Green Chemistry Chemistry Chemical Energy

Pin On Homeschool Body Anatomy Health

Pin By Margaret Casteel On School Structural Formula Chemistry Organic Chemistry
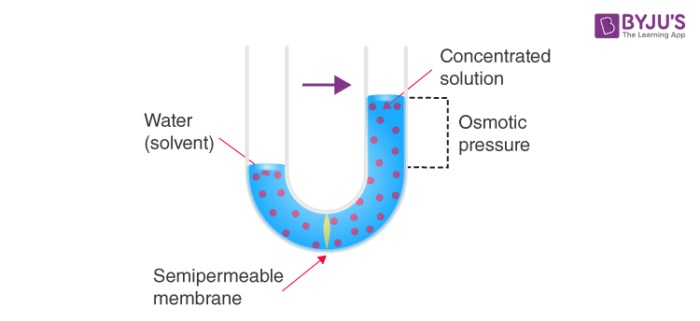
What Is Reverse Osmosis Ro Working Principle Water Purification
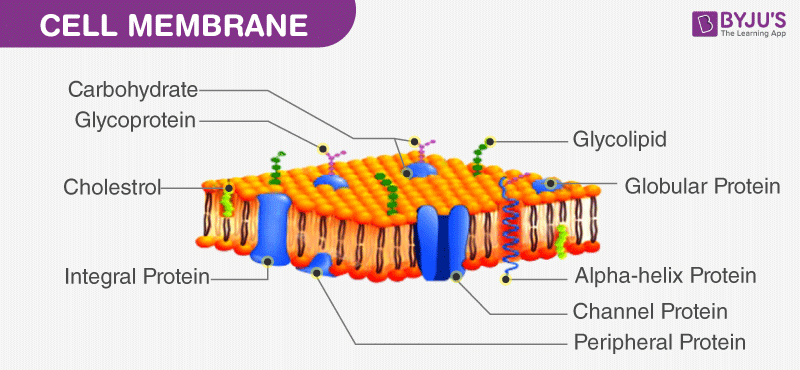
What Is Permeability Of Cell Membrane Byju S Neet

Boyle S Law Boyle S Law Law Chemistry

Quick Guide To Omega 3 6 9 Omega Fatty Acids Healthy Nutrition

Brain Important Diagrams For Cbse Class 10 Biology Biology Diagrams Basic Anatomy And Physiology Class 10
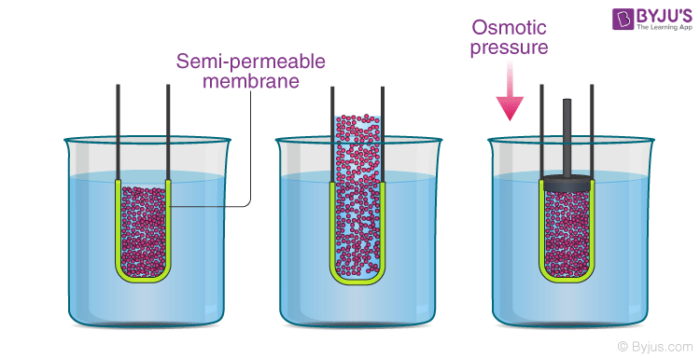
Osmotic Pressure Definition Formula Examples Solved Exercises

Hydrogen Isotopes Atomic Structure Atomic Theory Protons
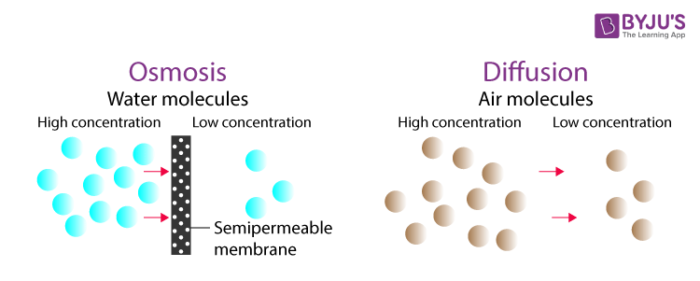
Diffusion Osmosis Difference Between Diffusion Osmosis
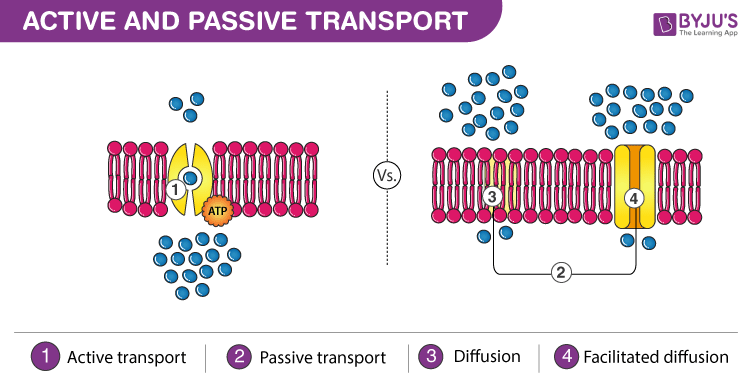
Difference Between Active Transport And Passive Transport

Potassium Iodate Structure Molecular Molecular Mass Potassium

Difference Between Osmosis And Diffusion In Tabular Form

Introduction To The World Of Cells Class 6 10 Learn With Byju S Youtube
Post a Comment for "What Is Osmosis Byju\'s"